Cell injury refers to a variety of processes that can cause damage to a cell, potentially leading to its dysfunction or death. It can result from a wide range of factors, including physical, chemical, infectious, immunologic, nutritional, and genetic causes.
when a cell is subjected to such extreme stress that it loses its ability to adapt or when cells are exposed to inherently damaging agents or suffer from intrinsic abnormalities. Various harmful stressors impact numerous cellular organelles and metabolic processes.
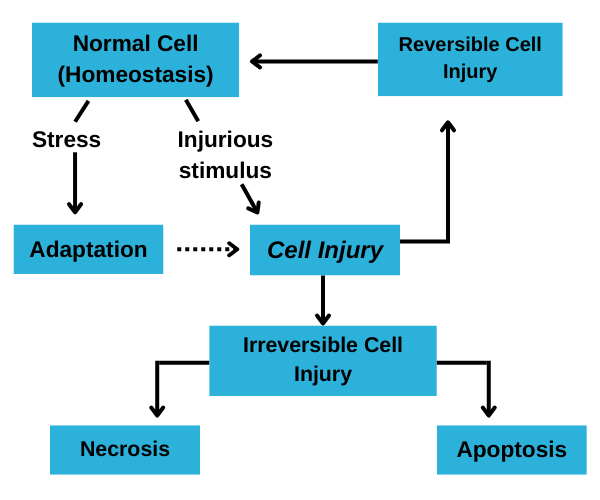
Types of Cell Injury:
Reversible Injury: This occurs when the damage to the cell is mild or transient, allowing the cell to recover if the injurious stimulus is removed.
Common features include:
- Cellular swelling
- Fatty change
- Plasma membrane alterations
- Mitochondrial changes
- Dilation of the endoplasmic reticulum
- Nuclear alterations
Irreversible Injury: When the damage is severe or prolonged, the cell may suffer irreversible injury leading to cell death.
There are two main ways in which this can occur:
Necrosis: Uncontrolled cell death resulting from severe damage. It often triggers an inflammatory response and can cause significant damage to surrounding tissues.
Apoptosis: Programmed cell death that occurs in a regulated manner. It helps eliminate damaged or unnecessary cells without causing inflammation.
Etiology of cell injury:
Hypoxia and Ischemia: Reduced oxygen supply (hypoxia) or blood flow (ischemia) can impair cellular respiration and energy production, leading to ATP depletion and subsequent cell injury.
Chemical Agents: Various chemicals and drugs can cause cell injury by interacting with cellular components. This includes toxins, environmental pollutants, and therapeutic drugs.
Infectious Agents: Bacteria, viruses, fungi, and parasites can cause cell injury by direct invasion, producing toxins, or eliciting immune responses that damage host tissues.
Immunologic Reactions: The immune system can inadvertently cause cell injury during hypersensitivity reactions, autoimmune diseases, or chronic inflammation.
Genetic Factors: Mutations or inherited disorders can lead to cell injury by producing abnormal proteins, disrupting normal cellular functions, or impairing metabolic pathways.
Nutritional Imbalances: Deficiencies or excesses of essential nutrients can impair cellular function and cause injury. This includes vitamin deficiencies, malnutrition, and obesity.
Physical Agents: Trauma, temperature extremes, radiation, and pressure changes can directly damage cellular structures and functions.
Aging: Cellular aging involves a decline in cellular function and regenerative capacity, making cells more susceptible to injury.
Pathogenesis of cell injury:
The pathogenesis of cell injury involves a complex sequence of events and mechanisms that lead to cellular dysfunction and ultimately cell death if the injury is severe or prolonged. Understanding these mechanisms is crucial for identifying potential therapeutic targets and interventions.
The primary targets of harmful stimuli are:
- Mitochondria, the sites of ATP generation,
Cell membranes, on which the iconic and osmotic homeostasis of the cell and its organelles depends:
- Protein synthesis,
- The cytoskeleton,
- The genetic apparatus of the cell.
A)Mechanisms of Reversible Cell Injury:
Cellular Swelling:
Mechanism: Due to failure of the sodium-potassium (Na+/K+) pump in the plasma membrane, which normally requires ATP to function. When ATP production is compromised (e.g., due to hypoxia), sodium accumulates inside the cell, leading to water influx and cellular swelling.
Morphological Changes: Increased cell volume, distended endoplasmic reticulum, and enlarged mitochondria. The plasma membrane may show blebbing.
Fatty Change (Steatosis):
Mechanism: Disruption in lipid metabolism, often seen in cells involved in fat metabolism such as hepatocytes. This can result from hypoxia, toxins, or metabolic disorders, leading to the accumulation of triglycerides within the cell.
Morphological Changes: Presence of lipid vacuoles in the cytoplasm, primarily in the liver, heart, and kidneys.
Depletion of ATP:
Mechanism: ATP depletion is a common feature of reversible cell injury and can result from reduced oxygen supply, mitochondrial damage, or inhibition of oxidative phosphorylation.
Consequences: Impaired function of ATP-dependent pumps (e.g., Na+/K+ pump), leading to ionic imbalance, cellular swelling, and disrupted protein synthesis.
Mitochondrial Changes:
Mechanism: Mitochondria may become swollen due to failure of ATP synthesis, leading to increased permeability and altered membrane potential.
Morphological Changes: Swollen mitochondria with distorted cristae, but no significant release of pro-apoptotic factors.
Dilation of the Endoplasmic Reticulum (ER):
Mechanism: Stress on the ER due to accumulation of misfolded proteins or disturbed calcium homeostasis.
Morphological Changes: Detachment of ribosomes from the rough ER and dilation of the ER cisternae.
Altered Membrane Permeability:
Mechanism: Injury can lead to increased permeability of the plasma membrane, allowing influx of calcium, sodium, and water, and efflux of potassium and small molecules.
Consequences: Ionic imbalance, cellular swelling, and possible activation of destructive enzymes.
Increased Anaerobic Glycolysis:
Mechanism: With impaired oxidative phosphorylation, cells switch to anaerobic glycolysis to generate ATP, leading to the production of lactic acid.
Consequences: Decreased pH (acidosis) and decreased glycogen stores.
Detachment of Ribosomes:
Mechanism: Due to energy depletion and stress, ribosomes may detach from the rough ER, leading to a decrease in protein synthesis.
Consequences: Reduced synthesis of proteins, including those required for cell repair and membrane integrity.
B)Mechanisms of Irreversible Cell Injury:
Mitochondrial Dysfunction:
Mechanism: Severe damage to mitochondria results in the inability to generate ATP, leading to energy failure. The loss of mitochondrial membrane potential also causes the release of pro-apoptotic proteins such as cytochrome c into the cytoplasm.
Consequences: Activation of the intrinsic pathway of apoptosis, disruption of ATP production, and initiation of cell death pathways.
Loss of Membrane Integrity:
Mechanism: Persistent or severe injury leads to the breakdown of plasma and organelle membranes. This can be due to direct damage by toxins, reactive oxygen species (ROS), or phospholipases.
Consequences: Leakage of cellular contents, loss of essential ions and metabolites, influx of calcium, and further activation of degradative enzymes (e.g., proteases, phospholipases, endonucleases).
Calcium Influx and Overload:
Mechanism: Sustained increase in intracellular calcium levels due to disrupted calcium homeostasis. High intracellular calcium activates a series of harmful enzymatic pathways.
Consequences: Activation of phospholipases (membrane damage), proteases (cytoskeletal degradation), endonucleases (DNA fragmentation), and ATPases (further depletion of ATP).
Reactive Oxygen Species (ROS) Generation:
Mechanism: Excessive generation of ROS overwhelms the cell’s antioxidant defenses. ROS can originate from mitochondria, peroxisomes, or inflammatory cells.
Consequences: Oxidative damage to lipids, proteins, and nucleic acids, leading to membrane damage, enzyme inactivation, and genetic mutations.
Irreversible Damage to DNA and Proteins:
Mechanism: Severe damage to DNA (e.g., fragmentation, mutations) and proteins (e.g., misfolding, cross-linking) that cannot be repaired.
Consequences: Activation of cell death pathways (e.g., p53-mediated apoptosis) and loss of essential cellular functions.
Lysosomal Membrane Damage:
Mechanism: Damage to lysosomal membranes results in the release of hydrolytic enzymes into the cytoplasm.
Consequences: Enzymatic digestion of cellular components and further degradation of cellular structures.
